
WithinĪ second-order perturbative approach, these contributions manifest as anĮffective intermolecular exchange interaction. Spin-orbit field acting on molecule B is generated by the electric fieldĪrising from charge density fluctuations in molecule A (and viceversa). Model assumes that the same fluctuating electric fields responsible for vdWįorces can induce a magnetic response via a Rashba-like term, so that an This investigation to describe the mutual induction of charge and spin-densityįluctuations in a pair A-B of chiral molecules by a simple physical model. Relation between spin and molecular geometry. The discovery of theĬhirality-Induced Spin Selectivity (CISS) effect in recent years has led to anĪdditional twist in the study of chiral molecular systems, showing a close Same distance scaling law R-6 as the London energy.
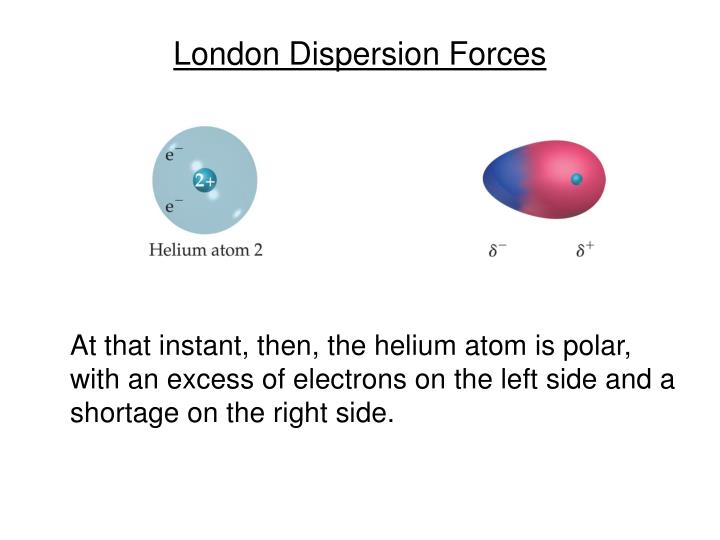
To the rotatory power known from the theory of circular dichroism and with the There are chirality-sensitive corrections to the dispersion forces proportional The occurrence of these short-range interactions is due to the fact that any atom will, at any given instant, be likely to possess a finite dipole moment as a result of the movement of electrons around the nuclei. Read reviews from worlds largest community for readers. Van der Waals or London dispersion forces are the universal forces responsible for attractive interactions between nonpolar molecules. Molecules lacking an inversion center such as chiral and helical molecules, London Dispersion Forces in Molecules, Solids and Nano-Structures book. However, it was only in the 1960s that it was recognized that for
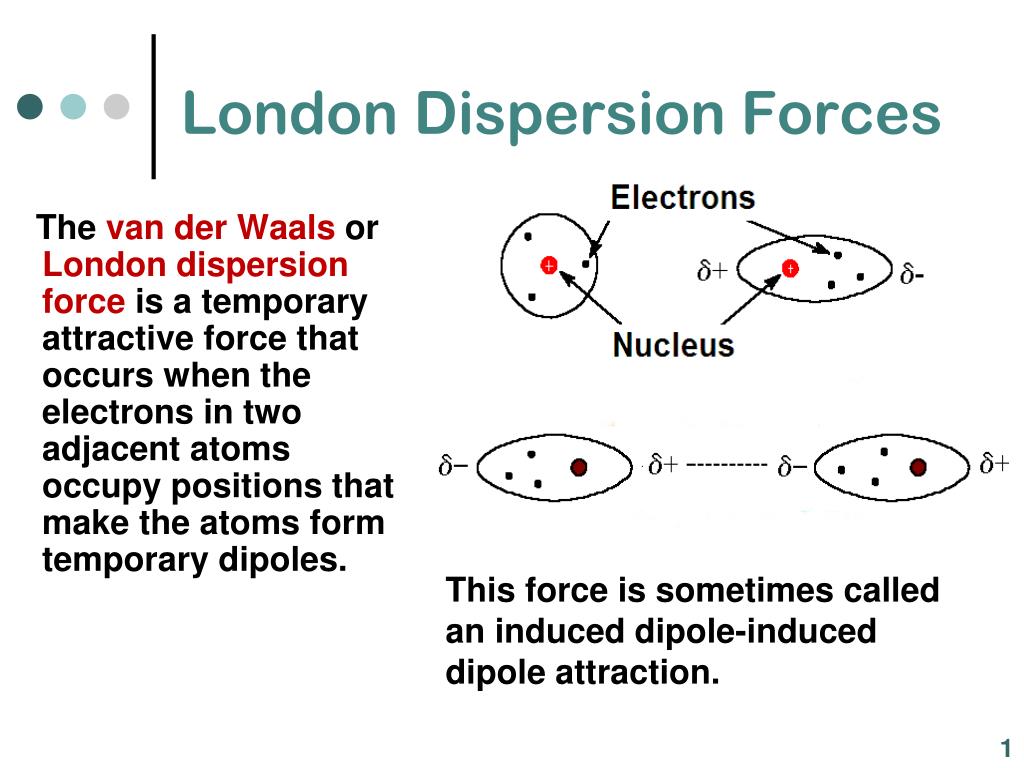
The theory ofĭispersion forces was developed by London in the early years of quantum They can then attract to each other in a. Interactions in many physical, chemical and biological processes. This instantaneous dipole can induce a dipole in another nearby non-polar molecule.
#London dispersio forces pdf
Cuniberti Download PDF Abstract: Dispersion interactions are one of the components of van der Waals (vdW)įorces, which play a key role in the understanding of intermolecular


 0 kommentar(er)
0 kommentar(er)
